The Resistance to Mass Transfer in the Stationary Phase
Principles and Practice of Chromatography
by RPW Scott
part of the Chrom Ed. Series
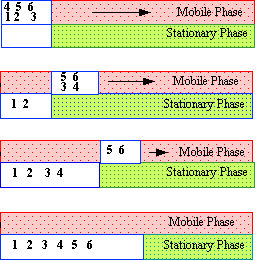
Figure 22 Resistance to Mass Transfer in the Mobile Phase
Van Deemter derived the following expression for the variance contribution by the resistance to mass transfer in the mobile phase, (),
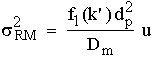 (6)
(6)
where (k’) is the capacity ratio of the solute, and the other symbols have the meaning previously ascribed to them.
The Resistance to Mass Transfer in the Stationary Phase
Dispersion due to resistance to mass transfer in the stationary phase is exactly analogous to that in the mobile phase. Solute molecules close to the interface will leave the stationary phase and enter the mobile phase before those that have diffused further into the stationary phase and have a longer distance to diffuse back. Thus, as those molecules that were close to the surface will be swept along in the moving phase, they will be dispersed from those molecules still diffusing to the surface. The dispersion resulting from the resistance to mass transfer in the stationary phase is depicted in figure 23. Molecules 1 and 2 (the two closest to the surface) will enter the mobile phase and begin moving along the column. Their movement will continue while molecules 3 and 4 diffuse to the interface at which time they will enter the mobile phase and start following molecules 1 and 2 down the column.
